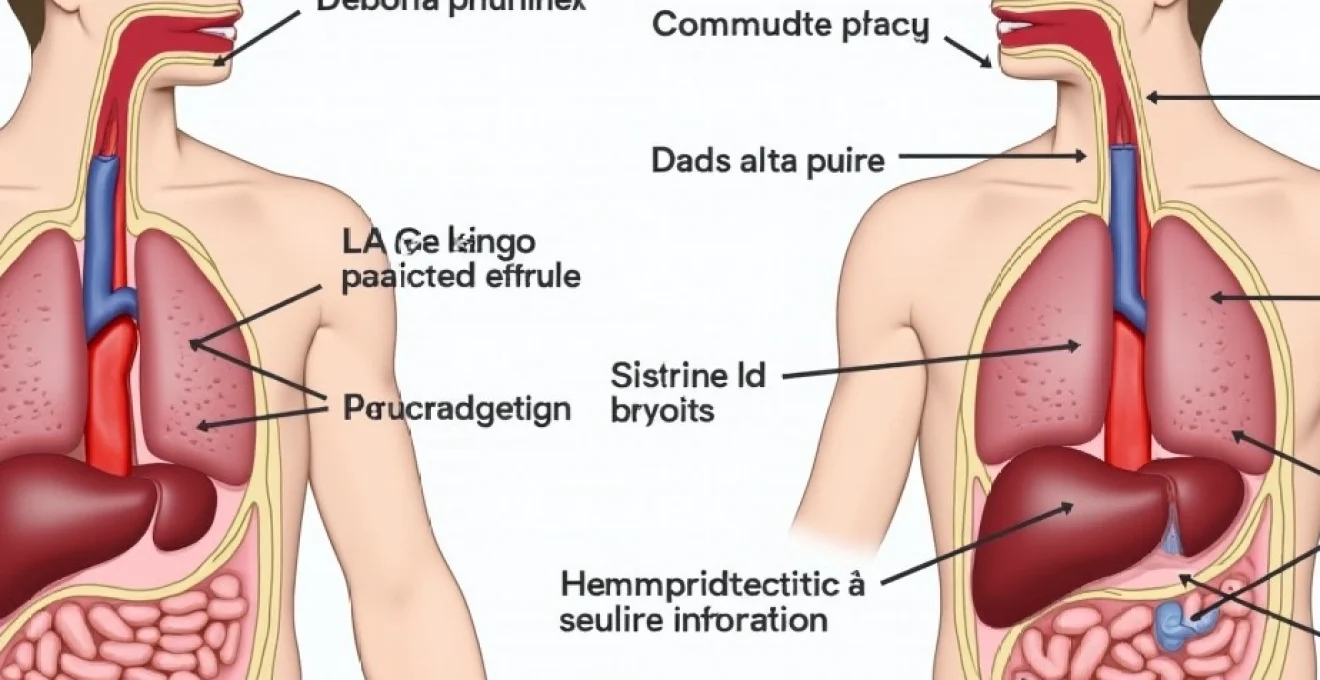
Chest pain that worsens when leaning forward represents a distinct clinical presentation that can indicate several underlying pathological processes. This positional component provides crucial diagnostic clues that help differentiate between various causes of thoracic discomfort. While many individuals immediately associate chest pain with cardiac conditions, the relationship between body position and pain intensity often points to specific anatomical structures and disease processes that extend beyond simple myocardial pathology.
Understanding the mechanisms behind position-dependent chest pain requires knowledge of thoracic anatomy and the various systems that can produce referred or localised discomfort. The forward-leaning position affects intrathoracic pressure, pericardial tension, pleural dynamics, and gastroesophageal anatomy in ways that can either exacerbate or alleviate symptoms depending on the underlying condition. This biomechanical relationship between posture and pain provides healthcare professionals with valuable diagnostic information that can guide appropriate investigation and treatment strategies.
Cardiac etiology of positional chest pain: pericardial and myocardial conditions
Cardiac causes of positional chest pain predominantly involve the pericardium and surrounding structures rather than primary myocardial pathology. The pericardium’s anatomy and physiology make it particularly sensitive to positional changes, creating characteristic pain patterns that serve as important diagnostic markers. When inflammation or fluid accumulation affects this protective cardiac membrane, patients often experience sharp, stabbing pain that demonstrates clear positional relationships.
Acute pericarditis and characteristic positional pain patterns
Acute pericarditis represents the most common cardiac cause of chest pain that improves with forward leaning. This inflammatory condition affects the pericardial layers, creating friction between the visceral and parietal surfaces during cardiac motion. The characteristic pain typically presents as sharp or stabbing discomfort located retrosternally or over the left chest, with radiation patterns extending to the shoulders, neck, or trapezius regions.
The pathophysiology behind pericarditic pain involves inflammatory mediators and mechanical irritation of pain-sensitive pericardial tissue. Forward leaning reduces pericardial tension and minimises friction between inflamed surfaces, explaining why patients instinctively adopt this position for relief. The pain classically worsens with inspiration, coughing, or lying supine, creating a distinctive clinical pattern that experienced clinicians readily recognise.
Viral infections represent the most frequent trigger for acute pericarditis in developed countries, though many cases remain idiopathic. Post-infectious inflammatory responses can persist for weeks following the initial viral illness, creating a delayed presentation that may confuse the clinical picture. Associated symptoms often include low-grade fever, malaise, and a characteristic three-component friction rub audible on cardiac examination.
Pericardial effusion with tamponade physiology
Pericardial effusion develops when fluid accumulates within the pericardial space, potentially progressing to cardiac tamponade if the accumulation rate exceeds the pericardium’s ability to stretch. The chest pain associated with pericardial effusion often demonstrates positional characteristics, though the predominant symptoms typically involve dyspnoea and haemodynamic compromise as the effusion progresses.
The forward-leaning position may provide temporary relief by optimising venous return and reducing the mechanical constraints imposed by pericardial fluid. However, as tamponade physiology develops, positional manoeuvres become less effective, and patients require urgent intervention. Beck’s triad of elevated jugular venous pressure, hypotension, and muffled heart sounds represents the classic presentation, though this complete constellation appears in fewer than 10% of cases.
Clinical recognition of pericardial effusion requires high index of suspicion, as the condition can rapidly progress from compensated physiology to life-threatening tamponade within hours.
Constrictive pericarditis and ventricular filling impairment
Constrictive pericarditis represents a chronic condition where pericardial scarring and calcification create a rigid shell around the heart, impairing ventricular filling and reducing cardiac output. Unlike acute pericarditis, constrictive disease rarely produces the classic positional pain patterns, instead manifesting with symptoms of right heart failure and exercise intolerance.
The chest discomfort associated with constrictive pericarditis tends to be more subtle and related to venous congestion rather than inflammatory pain. Patients may experience a sensation of chest fullness or pressure that correlates with elevated filling pressures and impaired ventricular compliance. The condition requires sophisticated imaging and haemodynamic assessment for accurate diagnosis and treatment planning.
Myocardial infarction with pericardial involvement
Post-infarction pericarditis, also known as Dressler syndrome, can develop days to weeks following myocardial infarction, creating chest pain with positional characteristics superimposed on the underlying coronary disease. This inflammatory response involves both the pericardium and pleura, producing pleuropericarditic pain that demonstrates typical positional relationships.
The incidence of post-infarction pericarditis has decreased significantly with modern reperfusion therapies, occurring in less than 5% of patients following acute myocardial infarction. When present, the condition typically responds well to anti-inflammatory therapy, though careful consideration must be given to bleeding risks in patients receiving anticoagulation for their coronary disease.
Pulmonary pathophysiology: pleuritic and parenchymal causes
Pulmonary causes of positional chest pain primarily involve the pleural surfaces and their interaction with respiratory mechanics. The pleura contains abundant sensory innervation that responds to inflammation, mechanical irritation, and changes in pleural pressure. When disease processes affect these structures, patients often experience pain that varies with respiratory effort and body position, creating characteristic clinical patterns that aid in differential diagnosis.
Pleuritis and pleural friction rub manifestations
Pleural inflammation, whether infectious or non-infectious in origin, commonly produces chest pain that worsens with movement and deep inspiration. The forward-leaning position may exacerbate pleuritic pain by increasing pleural surface contact and mechanical irritation during respiratory excursions. This contrasts with pericarditic pain, where forward leaning typically provides relief.
The characteristic sharp, stabbing nature of pleuritic pain results from direct stimulation of pleural sensory nerves during respiratory movement. Patients often describe the sensation as a knife-like pain that prevents deep breathing, leading to compensatory shallow respiratory patterns. The pain typically localises to the affected pleural surface, though referral patterns can occur based on the specific nerve pathways involved.
Viral pleuritis represents a common cause of isolated pleural pain, often occurring as part of a broader respiratory infection syndrome. The condition typically resolves spontaneously with supportive care, though the acute pain phase can be quite distressing for patients. Anti-inflammatory medications provide symptomatic relief while the underlying inflammatory process resolves.
Pneumothorax mechanical effects on thoracic pressure
Pneumothorax creates chest pain through mechanical disruption of normal pleural dynamics and altered thoracic pressure relationships. The pain associated with spontaneous pneumothorax typically presents acutely with sharp, unilateral chest discomfort accompanied by dyspnoea. Positional changes may affect the pain intensity by altering the distribution of intrapleural air and mechanical stress on pleural surfaces.
Primary spontaneous pneumothorax most commonly affects young, tall, thin males without underlying lung disease, often occurring during periods of physical exertion or changes in atmospheric pressure. The condition results from rupture of subpleural blebs or bullae, creating communication between the alveolar space and pleural cavity. Secondary pneumothorax occurs in patients with underlying lung disease and carries a higher risk of complications and recurrence.
The forward-leaning position may provide some comfort by reducing chest wall tension and optimising respiratory mechanics in the presence of pleural air. However, the primary therapeutic intervention involves pleural drainage for significant pneumothoraces, particularly those causing haemodynamic compromise or respiratory distress.
Pulmonary embolism with pleural surface irritation
Pulmonary embolism can produce chest pain through multiple mechanisms, including pleural irritation when peripheral emboli cause pulmonary infarction. The chest pain associated with pulmonary embolism often demonstrates pleuritic characteristics, worsening with inspiration and movement. Forward leaning may exacerbate the discomfort by increasing pleural surface contact during respiratory excursions.
The clinical presentation of pulmonary embolism varies considerably based on the size and location of the embolic burden. Massive pulmonary embolism typically presents with haemodynamic instability and right heart strain, while smaller peripheral emboli may produce isolated chest pain and dyspnoea. The Wells score and other clinical prediction rules help stratify patients for appropriate diagnostic testing.
Pulmonary embolism remains a diagnostic challenge due to its non-specific presentation and the need for timely recognition to prevent potentially fatal outcomes.
D-dimer testing provides a useful screening tool in low-probability patients, though elevated levels occur in numerous conditions and limit specificity. CT pulmonary angiography represents the gold standard for diagnosis in most clinical situations, providing detailed visualisation of pulmonary vasculature and alternative diagnoses when embolism is not present.
Pneumonia-induced pleuritic pain mechanisms
Pneumonia can produce chest pain through inflammatory involvement of the pleura, particularly when the infection extends to the peripheral lung parenchyma. The pain typically demonstrates pleuritic characteristics, with sharp, localised discomfort that worsens with respiratory effort. The forward-leaning position may increase discomfort by promoting deeper respiratory excursions and greater pleural surface irritation.
Community-acquired pneumonia affects approximately 5-6 individuals per 1000 adults annually, with Streptococcus pneumoniae representing the most common bacterial pathogen. Atypical pathogens such as Mycoplasma pneumoniae and viral causes may produce less pronounced pleural involvement, resulting in more subtle chest discomfort patterns.
The diagnostic approach to pneumonia-related chest pain involves chest radiography, which demonstrates characteristic infiltrates in most cases of bacterial pneumonia. However, early disease or infection with atypical pathogens may present with normal initial imaging, requiring clinical correlation and potentially advanced imaging techniques for accurate diagnosis.
Gastrointestinal referred pain: oesophageal and upper abdominal origins
Gastrointestinal causes of chest pain frequently demonstrate positional relationships due to anatomical proximity and shared nerve pathways between thoracic and abdominal structures. The oesophagus, in particular, lies in intimate contact with the posterior pericardium and can produce chest discomfort that mimics cardiac pain. Understanding these referral patterns and positional relationships helps clinicians differentiate between cardiac and gastrointestinal causes of thoracic symptoms.
Gastroesophageal reflux disease (GORD) positional exacerbation
Gastroesophageal reflux disease represents one of the most common causes of non-cardiac chest pain, affecting up to 20% of adults in developed countries. The chest pain associated with GORD typically worsens with forward leaning due to gravitational effects on gastric contents and increased intra-abdominal pressure. This positional relationship provides an important diagnostic clue that differentiates GORD-related pain from pericarditic pain, which improves with forward leaning.
The pathophysiology of GORD-related chest pain involves acid-induced inflammation of the oesophageal mucosa and stimulation of visceral pain receptors. The shared embryological origins of cardiac and oesophageal tissues result in similar sensory nerve pathways, explaining why oesophageal pain can closely mimic cardiac angina. This visceral convergence creates significant diagnostic challenges in clinical practice.
Postprandial timing represents another important characteristic of GORD-related chest pain, with symptoms typically occurring 30-60 minutes after meals. The pain often accompanies other reflux symptoms such as heartburn, regurgitation, and water brash, though isolated chest pain can occur without classic reflux symptoms in approximately 25% of cases.
Lifestyle modifications form the cornerstone of GORD management, including dietary changes, weight loss, and positional therapy during sleep. Proton pump inhibitors provide effective acid suppression and symptom relief in most patients, though long-term use requires consideration of potential adverse effects including increased fracture risk and drug interactions.
Oesophageal spasm and motility disorders
Oesophageal motility disorders can produce severe chest pain that may be difficult to distinguish from cardiac causes without sophisticated diagnostic testing. Diffuse oesophageal spasm creates intense, cramping chest pain that may last minutes to hours and can be triggered by swallowing, emotional stress, or temperature changes. The pain rarely demonstrates consistent positional relationships, making clinical diagnosis challenging.
The pain mechanism involves abnormal coordinated contractions of oesophageal smooth muscle, creating elevated intraluminal pressures and visceral pain sensation. High-resolution oesophageal manometry provides definitive diagnosis by demonstrating characteristic pressure patterns and contractile abnormalities throughout the oesophageal length.
Nutcracker oesophagus, characterised by high-amplitude peristaltic contractions, can produce similar chest pain symptoms though the contractions remain coordinated. Treatment approaches include smooth muscle relaxants, calcium channel blockers, and in severe cases, interventional procedures such as pneumatic dilatation or surgical myotomy.
Hiatal hernia anatomical displacement effects
Hiatal hernia involves displacement of gastric contents into the thoracic cavity through the oesophageal hiatus, potentially creating chest discomfort through multiple mechanisms. Large hiatal hernias can produce mechanical chest pain due to mass effect and altered thoracic anatomy, while associated gastroesophageal reflux contributes to chemical irritation and inflammation.
Paraesophageal hiatal hernias represent a more severe variant where portions of the stomach herniate alongside the oesophagus, potentially causing gastric volvulus and ischaemia. These large hernias can produce postural chest pain that worsens with recumbency and may improve with upright positioning, creating a pattern opposite to typical GORD symptoms.
The forward-leaning position may exacerbate symptoms by increasing intra-abdominal pressure and promoting further gastric displacement into the thorax. Surgical repair becomes necessary for large symptomatic hiatal hernias, particularly those causing recurrent symptoms or complications such as gastric outlet obstruction.
Peptic ulcer disease referred pain patterns
Peptic ulcer disease can occasionally produce referred chest pain, particularly when ulcers involve the posterior duodenal wall or gastric fundus. The pain typically demonstrates relationships to meal timing and may be accompanied by classic dyspeptic symptoms such as epigastric discomfort, bloating, and nausea. Forward leaning may exacerbate symptoms by increasing gastric pressure and promoting acid secretion.
Helicobacter pylori infection remains the predominant cause of peptic ulcer disease worldwide, affecting approximately 50% of the global population. The organism’s urease activity creates local alkalinity that allows survival in the acidic gastric environment, leading to chronic inflammation and epithelial damage. Eradication therapy typically involves triple or quadruple antibiotic regimens combined with acid suppression.
Modern peptic ulcer management has shifted dramatically from surgical intervention to medical therapy, with cure rates exceeding 95% when appropriate eradication protocols are followed.
Musculoskeletal differential diagnosis: thoracic wall and spinal pathology
Musculoskeletal causes of chest pain often demonstrate clear positional relationships due to the mechanical nature of the underlying pathology. The chest wall contains multiple anatomical structures capable of producing pain, including the sternum, ribs, costal cartilages, intercostal muscles, and thoracic spine. When these structures become inflamed, injured, or mechanically compromised, patients frequently experience pain that varies with position, movement, and respiratory effort.
Costochondritis represents one of the most common musculoskeletal causes of chest pain, involving inflammation of the costal cartilages where ribs attach to the sternum. The condition typically affects the second through fifth costochondral junctions and produces sharp, stabbing pain that worsens with movement, coughing, or deep inspiration
. The forward-leaning position may exacerbate costochondritis pain by stretching the inflamed cartilaginous structures and increasing mechanical stress on the affected joints. Palpation of the costochondral junctions typically reproduces the pain, providing a diagnostic clue that distinguishes this condition from cardiac causes of chest discomfort.
Thoracic spine pathology can produce referred chest pain through complex neural pathways connecting spinal segments to anterior chest wall structures. Thoracic disc herniation, facet joint arthritis, and vertebral compression fractures may all contribute to chest wall pain that demonstrates positional characteristics. The forward-leaning position often exacerbates thoracic spine-related pain by increasing spinal flexion and loading on affected structures.
Intercostal muscle strain represents another common musculoskeletal cause of position-dependent chest pain, often resulting from sudden movements, repetitive activities, or direct trauma. The pain typically follows the distribution of specific intercostal nerves and worsens with respiratory effort or trunk rotation. Trigger points within intercostal muscles can produce localised tenderness and referred pain patterns that may confuse the clinical presentation.
Rib fractures, whether traumatic or pathological, create characteristic chest wall pain that demonstrates clear mechanical relationships to movement and position. The forward-leaning position may increase pain by altering chest wall mechanics and placing additional stress on the fracture site. Elderly patients with osteoporosis face particular risk for spontaneous rib fractures during minimal trauma or even vigorous coughing episodes.
Red flag symptoms requiring immediate medical assessment
Certain clinical presentations demand immediate medical evaluation regardless of positional pain characteristics, as they may indicate life-threatening conditions requiring urgent intervention. These red flag symptoms help clinicians rapidly identify patients who need emergency care rather than routine diagnostic workups. Understanding these warning signs enables both healthcare professionals and patients to recognise when chest pain represents a medical emergency.
Chest pain accompanied by haemodynamic instability, including hypotension, tachycardia, or signs of shock, requires immediate evaluation for conditions such as myocardial infarction, pulmonary embolism, or aortic dissection. The combination of severe chest pain with syncope or near-syncope suggests massive pulmonary embolism, acute coronary syndrome, or cardiac arrhythmias that demand urgent treatment. These presentations override considerations of positional pain characteristics and require immediate stabilisation and diagnostic intervention.
Chest pain with associated neurological symptoms, including focal weakness, speech difficulties, or altered mental status, may indicate stroke, particularly when accompanied by neck or jaw pain suggestive of carotid or vertebral artery dissection. The simultaneous occurrence of chest and neurological symptoms creates a complex clinical scenario requiring multidisciplinary evaluation and immediate imaging studies.
The presence of red flag symptoms transforms chest pain from a routine diagnostic challenge into a medical emergency requiring immediate intervention and multidisciplinary care coordination.
Chest pain accompanied by fever and signs of systemic toxicity raises concern for infectious processes such as pneumonia with sepsis, infective endocarditis, or mediastinitis. These conditions can rapidly progress to multi-organ failure and require prompt antibiotic therapy along with supportive care. The combination of positional chest pain with high fever and rigors particularly suggests pleural or pericardial infection requiring urgent drainage procedures.
Sudden onset of severe chest pain that reaches maximum intensity within seconds suggests catastrophic conditions such as aortic dissection, spontaneous pneumothorax, or acute coronary occlusion. This thunderclap onset pattern differs markedly from gradual onset inflammatory conditions and demands immediate evaluation with advanced imaging and cardiothoracic surgical consultation when indicated.
Diagnostic approach: clinical examination and investigative protocols
A systematic diagnostic approach to positional chest pain begins with comprehensive history-taking that explores the temporal relationship between symptoms and body position, associated triggers, and accompanying systemic symptoms. The clinical examination should focus on identifying specific physical findings that correlate with the patient’s positional pain complaints, using targeted manoeuvres to reproduce or alleviate symptoms under controlled conditions.
The cardiovascular examination requires particular attention to pericardial friction rubs, which create characteristic three-component sounds corresponding to atrial systole, ventricular systole, and ventricular diastole. These sounds may only be audible in specific positions, requiring examination in both supine and sitting positions to maximise detection sensitivity. The presence of elevated jugular venous pressure, hepatojugular reflux, or peripheral oedema suggests advanced pericardial disease or heart failure requiring urgent evaluation.
Pulmonary examination focuses on detecting pleural friction rubs, diminished breath sounds suggesting effusion or pneumothorax, and characteristic patterns of adventitious sounds that may indicate underlying parenchymal disease. The examination should include assessment of respiratory excursion symmetry and evaluation of tactile fremitus to identify pleural abnormalities that may contribute to positional pain patterns.
Musculoskeletal examination involves systematic palpation of chest wall structures, including costochondral junctions, intercostal spaces, and thoracic spine. Specific provocative manoeuvres, such as chest wall compression and spinal rotation, can help localise pain sources and differentiate musculoskeletal from visceral causes. The examiner should assess for point tenderness, muscle spasm, and reproducibility of the patient’s symptoms with specific movements or positions.
Initial investigations should be guided by clinical probability and may include electrocardiography to detect pericardial injury patterns, chest radiography to evaluate for pleural effusion or pneumothorax, and basic inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate. The ECG may demonstrate characteristic changes in pericarditis, including widespread ST elevation and PR depression, though these findings are present in fewer than 50% of cases.
Advanced imaging modalities play crucial roles when initial investigations are inconclusive or clinical suspicion remains high for specific conditions. Echocardiography provides excellent visualisation of pericardial effusion and can detect early signs of tamponade physiology through assessment of ventricular filling patterns and respiratory variation in flow velocities. CT scanning offers superior evaluation of mediastinal structures and can identify conditions such as aortic dissection or pulmonary embolism that may present with positional chest pain.
Laboratory investigations should be tailored to clinical suspicion but may include cardiac biomarkers when myocardial involvement is suspected, D-dimer levels for pulmonary embolism assessment, and specific inflammatory markers depending on the clinical scenario. Troponin elevation can occur in pericarditis due to epicardial involvement, though levels are typically lower than those seen in significant myocardial infarction.
The diagnostic approach must remain flexible and responsive to evolving clinical findings, as positional chest pain can represent the initial presentation of progressive conditions requiring serial assessment and investigation. Close follow-up arrangements ensure appropriate monitoring of symptom evolution and response to initial therapeutic interventions, allowing for diagnostic revision when clinical improvement does not occur as expected.