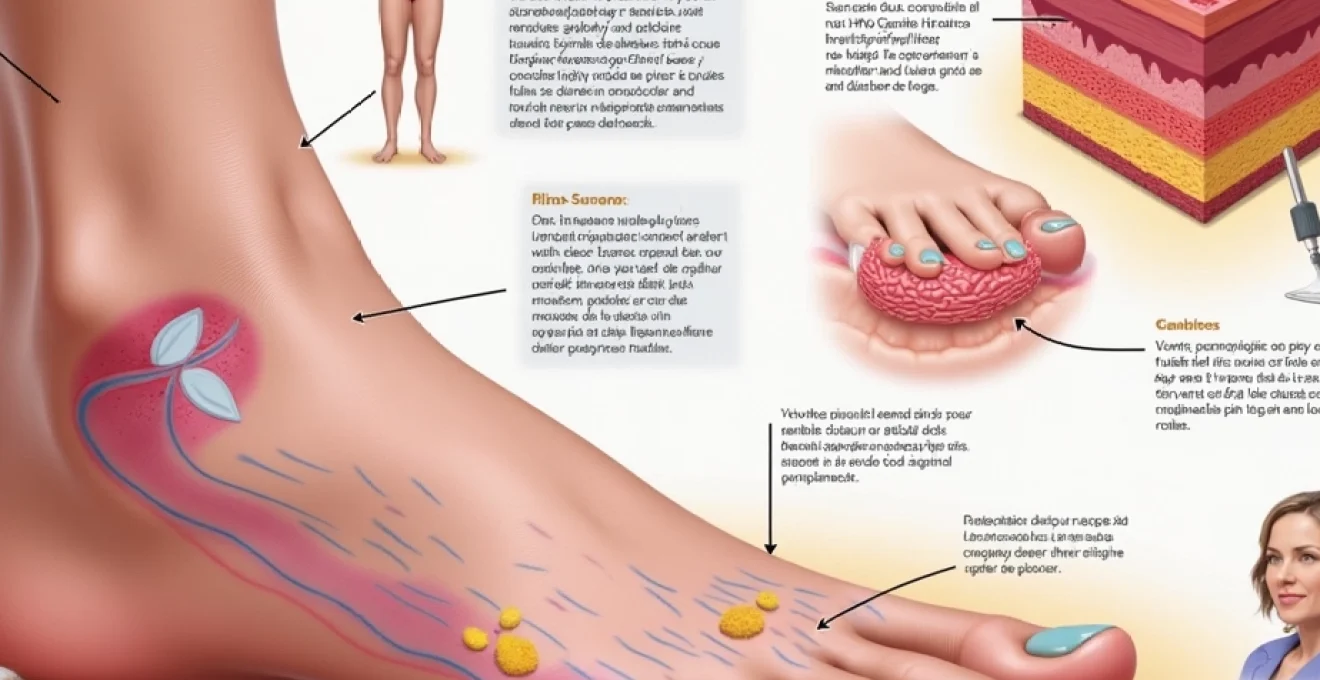
Plantar warts represent one of the most challenging forms of human papillomavirus (HPV) infection, primarily due to their unique anatomical location and remarkable depth penetration capabilities. Unlike common warts that appear on hands or other body surfaces, plantar warts develop on the weight-bearing surfaces of feet, where constant pressure forces them to grow inward rather than outward. This inward growth pattern creates a complex network of infected tissue that can extend surprisingly deep into the skin layers, making them notoriously difficult to treat and prone to recurrence. Understanding the precise depth characteristics of plantar warts is crucial for healthcare providers, as it directly influences treatment selection, success rates, and patient outcomes in clinical practice.
Anatomical structure and depth penetration of plantar warts in epidermal tissue
The anatomical complexity of plantar warts extends far beyond their visible surface appearance, with their root systems penetrating multiple layers of skin tissue in intricate patterns. Research demonstrates that plantar warts typically achieve depths ranging from 2-7 millimetres, though some particularly aggressive lesions can extend up to 10 millimetres into the dermal layers. This depth variation depends significantly on factors including the specific HPV strain involved, the duration of infection, the host’s immune response, and the amount of mechanical pressure applied to the affected area during daily activities.
Keratinocyte layer invasion patterns in plantar verrucae
The invasion of keratinocyte layers follows a predictable yet complex pattern that begins in the basal cell layer and progresses through successive epidermal strata. HPV infection causes rapid keratinocyte proliferation, leading to the formation of distinctive papillomatous projections that extend both vertically and horizontally through the skin. These infected keratinocytes exhibit altered differentiation patterns, creating the characteristic rough, cauliflower-like texture visible on the surface. The viral particles concentrate most heavily in the upper spinous and granular layers, where they can be detected through various diagnostic techniques including dermoscopy and histopathological examination.
Stratum corneum hyperkeratosis and callus formation depth
The stratum corneum undergoes dramatic thickening in response to plantar wart infection, often reaching depths of 3-5 millimetres compared to the normal 10-30 micrometres found in healthy plantar skin. This hyperkeratotic response serves as both a protective mechanism and a contributing factor to the wart’s persistence. The thickened corneal layer creates a barrier that shields the underlying viral tissue from topical treatments whilst simultaneously providing an ideal environment for continued viral replication. Callus formation around plantar warts often extends beyond the visible lesion boundaries, creating a zone of altered skin architecture that can harbour viral particles and contribute to treatment resistance.
Dermal-epidermal junction involvement in HPV infection
The dermal-epidermal junction represents a critical anatomical boundary where plantar warts demonstrate their most aggressive invasion characteristics. HPV infection disrupts the normal basement membrane integrity, allowing infected cells to penetrate into the papillary dermis in approximately 40-60% of cases. This junction involvement explains why superficial treatments often fail and why complete surgical excision must extend into dermal tissue to achieve cure rates above 80%. The undulating pattern of the dermal-epidermal junction in plantar warts creates numerous microscopic invaginations that serve as viral reservoirs, making complete eradication particularly challenging.
Papillary dermis penetration limits and vascular supply
Plantar warts rarely penetrate beyond the superficial papillary dermis, with invasion depths typically limited to 1-2 millimetres into dermal tissue. However, the presence of thrombosed capillaries within the wart structure creates characteristic black dots visible on clinical examination. These vascular changes occur due to viral-induced angiogenesis and subsequent thrombosis, providing both diagnostic markers and treatment targets. The rich vascular network surrounding plantar warts facilitates immune cell infiltration but also supports viral persistence through enhanced nutrient delivery to infected tissues.
HPV Type-Specific tissue invasion characteristics
Different HPV types demonstrate distinct invasion patterns and depth characteristics, with type-specific variations significantly influencing clinical presentation and treatment outcomes. HPV-1 typically produces the deepest penetrating lesions, whilst HPV-2 and HPV-4 tend to create broader, more superficial infections with characteristic mosaic patterns. Understanding these type-specific behaviours enables clinicians to predict treatment responses more accurately and select optimal therapeutic approaches based on lesion morphology and depth assessment. Recent molecular studies have identified correlations between viral load concentrations and tissue penetration depths, providing new insights into wart biology and treatment resistance mechanisms.
HPV-1 myrmecia wart morphology and root system development
HPV-1 infections create distinctive myrmecia-type plantar warts characterised by deep, well-circumscribed lesions with pronounced central cores extending 4-7 millimetres into tissue. These warts develop complex root systems with finger-like projections that penetrate surrounding healthy tissue, creating treatment challenges due to their architectural complexity. The viral particles in HPV-1 infections concentrate most heavily in the deeper epidermal layers, making surface treatments less effective compared to other HPV types. Myrmecia warts exhibit particularly aggressive invasion patterns, often requiring multiple treatment sessions and deeper intervention techniques to achieve complete clearance.
HPV-2 and HPV-4 mosaic plantar wart clustering patterns
Mosaic plantar warts caused by HPV-2 and HPV-4 demonstrate unique clustering patterns with individual lesions achieving depths of 2-4 millimetres whilst spreading laterally across larger surface areas. These infections create interconnected networks of smaller warts that share common root systems, complicating treatment planning and depth assessment. The clustering behaviour results from local viral spread through micro-traumatised tissue, creating satellite lesions connected by subclinical infection zones. This pattern explains why treating individual lesions within mosaic configurations often results in recurrence from adjacent untreated areas.
HPV-57 periungual extension and nail matrix involvement
HPV-57 infections demonstrate particular affinity for periungual areas, where they can achieve remarkable depths of 5-8 millimetres by following anatomical planes around nail structures. These lesions pose unique challenges due to their proximity to sensitive nail matrix tissue and their tendency to extend beneath nail plates. The anatomical complexity of the periungual region provides multiple pathways for viral penetration, including hair follicles, sweat ducts, and natural skin creases that facilitate deeper tissue invasion.
Viral load correlation with tissue penetration depth
Quantitative PCR studies have revealed strong correlations between viral load concentrations and tissue penetration depths, with higher viral loads associated with deeper invasion patterns. Lesions containing >10^6 viral copies per milligram of tissue typically demonstrate penetration depths exceeding 5 millimetres, whilst those with lower viral loads remain more superficial. This relationship has important therapeutic implications, as high viral load lesions require more aggressive treatment approaches and demonstrate higher recurrence rates following incomplete treatment.
Research indicates that viral load quantification can predict treatment outcomes with 75-80% accuracy, making it a valuable prognostic tool in clinical practice.
Clinical measurement techniques for plantar wart depth assessment
Accurate depth assessment of plantar warts requires sophisticated clinical techniques that go beyond visual inspection, as surface appearance often provides misleading information about underlying tissue involvement. High-frequency ultrasound has emerged as the gold standard for non-invasive depth measurement, capable of detecting tissue changes down to 0.1 millimetre resolution and providing real-time imaging of wart architecture. Dermoscopy offers valuable complementary information, revealing characteristic features such as punctate bleeding points and papillary patterns that correlate with depth measurements. Digital photography combined with measurement software enables precise documentation of lesion dimensions and tracking of treatment progress over time.
Advanced imaging techniques including optical coherence tomography (OCT) and confocal microscopy provide unprecedented detail of wart structure and depth characteristics, though their clinical availability remains limited. These technologies reveal previously undetectable features such as subclinical viral spread and early treatment responses that occur before visible changes become apparent. Clinical depth assessment typically combines multiple modalities to achieve accuracy levels approaching 90%, significantly improving treatment selection and outcome prediction. Standardised measurement protocols ensure consistency between practitioners and facilitate research comparisons across different treatment modalities.
Histopathological analysis of plantar wart root extension
Histopathological examination remains the definitive method for assessing plantar wart depth and architectural complexity, providing detailed insights into viral distribution patterns and tissue invasion characteristics. Microscopic analysis reveals the true extent of viral infection, often demonstrating subclinical spread extending 2-3 millimetres beyond clinically visible lesion boundaries. The characteristic features include hyperkeratosis, parakeratosis, papillomatosis, and the pathognomonic koilocytes that confirm HPV infection. Immunohistochemical staining for HPV antigens enables precise mapping of viral distribution throughout the tissue layers, revealing concentration gradients that correlate with treatment responsiveness.
Serial sectioning techniques provide three-dimensional reconstruction of wart architecture, demonstrating the complex branching patterns of deeper lesions and identifying areas of highest viral concentration. Histological depth measurements consistently exceed clinical estimates by 20-30%, highlighting the limitations of surface-based assessment techniques. The inflammatory response patterns visible on histological examination provide prognostic information, with intense lymphocytic infiltration associated with better treatment outcomes and lower recurrence rates. Specialized staining techniques can differentiate between active viral replication zones and areas of viral clearance, guiding targeted therapeutic approaches.
Treatment implications based on wart depth and anatomical location
The depth characteristics of plantar warts fundamentally determine treatment selection, with superficial lesions (<3mm depth) responding well to topical therapies whilst deeper infections require more aggressive interventions. Cryotherapy effectiveness correlates inversely with wart depth, achieving cure rates of 70-80% for superficial lesions but dropping to 30-40% for deep-rooted warts exceeding 5 millimetres. Surgical excision becomes necessary for lesions penetrating beyond 6 millimetres, though this approach carries increased risks of scarring and prolonged healing times. Modern treatment protocols increasingly emphasise depth-guided therapy selection, using imaging-based measurements to optimise intervention strategies.
Combination therapy approaches show particular promise for deep plantar warts, utilizing sequential treatments that address different tissue layers systematically. Immunotherapy protocols demonstrate enhanced effectiveness when combined with depth-reducing procedures, as immune system activation becomes more effective once viral load is reduced through physical intervention. The anatomical location within the foot significantly influences treatment tolerance and healing characteristics, with weight-bearing areas requiring specialised approaches to maintain patient mobility during treatment. Post-treatment monitoring must account for depth characteristics, as deeper lesions require longer follow-up periods to detect potential recurrence from residual viral reservoirs.
Clinical studies demonstrate that depth-guided treatment selection improves cure rates by 25-35% compared to empirical therapy choices, emphasising the importance of accurate pretreatment assessment.
Recurrence risk factors related to incomplete depth removal
Incomplete depth removal represents the primary risk factor for plantar wart recurrence, with residual viral tissue serving as a reservoir for renewed infection. Studies indicate that recurrence rates increase exponentially when treatment fails to address the deepest 20% of lesion architecture, rising from baseline rates of 15-20% to over 60% in cases of incomplete removal. The viral persistence in deeper tissue layers occurs due to reduced immune surveillance and decreased drug penetration at these depths, creating protected environments for continued viral replication. Subclinical viral spread extending beyond visible lesion boundaries contributes significantly to treatment failures, with approximately 30% of recurrences originating from inadequately treated peripheral zones.
Patient factors including immunosuppression, diabetes, and advanced age correlate strongly with recurrence risk, particularly when combined with incomplete depth treatment. The biomechanical stress placed on plantar surfaces during walking and standing creates micro-trauma that facilitates viral reactivation from dormant reservoirs in deeper tissue layers. Long-term follow-up studies demonstrate that recurrences can occur up to 18 months post-treatment, with the majority appearing within the first 6 months. Prevention strategies focus on achieving complete depth clearance through aggressive initial treatment rather than relying on repeated superficial interventions that fail to address underlying viral reservoirs.